Liquifaction of Gases :
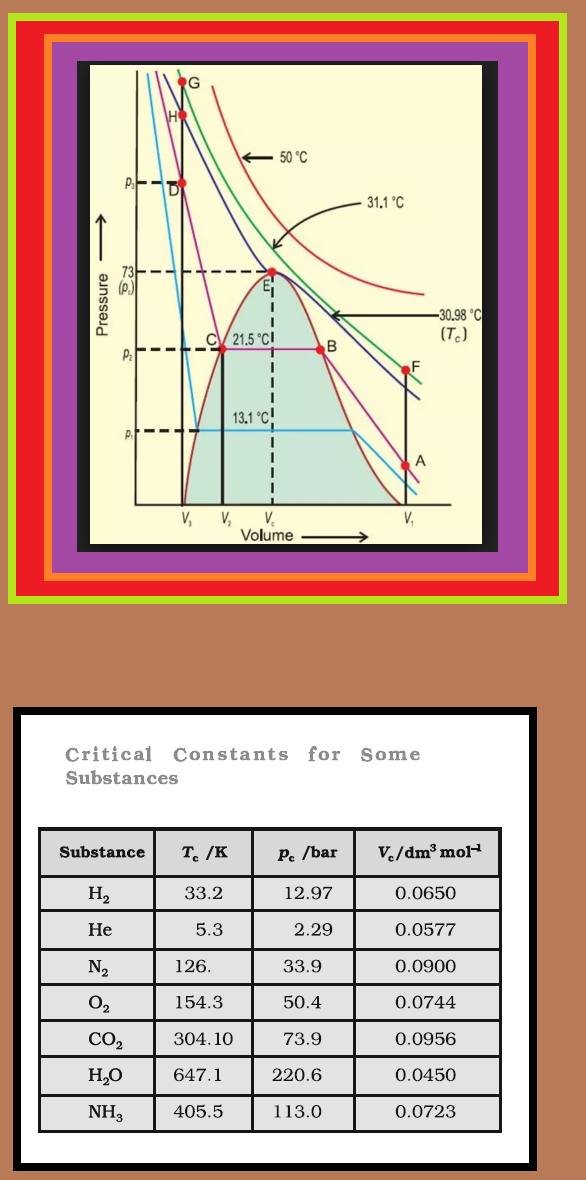
`=>` First complete data on pressure - volume - temperature relations of a substance in both gaseous and liquid state was obtained by Thomas Andrews on carbon dioxide.
● He plotted isotherms of carbon dioxide at various temperatures (Fig.).
● Later on it was found that real gases behave in the same manner as carbon dioxide.
● Andrews noticed that at high temperatures isotherms look like that of an ideal gas and the gas cannot be liquified even at very high pressure.
● As the temperature is lowered, shape of the curve changes and data shows considerable deviation from ideal behaviour.
● At `30.98 °C` carbon dioxide remains gas up to `73` atmospheric pressure. (Point `E` in Fig.). At `73` atmospheric pressure, liquid carbon dioxide appears for the first time. The temperature `30.98 °C` is called `text(critical temperature)` (`T_C`) of carbon dioxide. This is the highest temperature at which liquid carbon dioxide is observed. Above this temperature it is gas. Volume of one mole of the gas at critical temperature is called `text(critical volume)` (`V_C`) and pressure at this temperature is called `text(critical pressure)` (`p_C`). The critical temperature, pressure and volume are called critical constants.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Critical temperature of a gas may be defined as that temperature above which it cannot be liquified howsoever high pressure may be applied on the gas.
● Further increase in pressure simply compresses the liquid carbon dioxide and the curve represents the compressibility of the liquid. The steep line represents the isotherm of liquid. Even a slight compression results in steep rise in pressure indicating very low compressibility of the liquid.
● Below `30.98 °C`, the behaviour of the gas on compression is quite different.
● At `21.5 °C`, carbon dioxide remains as a gas only up to point `B`. At point `B`, liquid of a particular volume appears.
● Further compression does not change the pressure. Liquid and gaseous carbon dioxide coexist and further application of pressure results in the condensation of more gas until the point `C` is reached.
● At point `C`, all the gas has been condensed and further application of pressure merely compresses the liquid as shown by steep line.
● A slight compression from volume `V_2` to `V_3` results in steep rise in pressure from `p_2` to `p_3` (Fig. 5.11).
● Below `30.98 °C` (critical temperature) each curve shows the similar trend. Only length of the horizontal line increases at lower temperatures.
● At critical point horizontal portion of the isotherm merges into one point.
● Thus we see that a point like `A` in the Fig. represents gaseous state. A point like `D` represents liquid state and a point under the dome shaped area represents existence of liquid and gaseous carbon dioxide in equilibrium.
● All the gases upon compression at constant temperature (isothermal compression) show the same behaviour as shown by carbon dioxide.
● Also above discussion shows that gases should be cooled below their critical temperature for liquification.
● Critical temperature of a gas is highest temperature at which liquifaction of the gas first occurs.
● Liquifaction of so called permanent gases (i.e., gases which show continuous positive deviation in Z value) requires cooling as well as considerable compression. Compression brings the molecules in close vicinity and cooling slows down the movement of molecules therefore, intermolecular interactions may hold the closely and slowly moving molecules together and the gas liquifies.
● It is possible to change a gas into liquid or a liquid into gas by a process in which always a single phase is present.
● For example in Fig we can move from point `A` to `F` vertically by increasing the temperature, then we can reach the point `G` by compressing the gas at the constant temperature along this isotherm (isotherm at `31.1°C`). The pressure will increase. Now we can move vertically down towards `D` by lowering the temperature. As soon as we cross the point `H` on the critical isotherm we get liquid. We end up with liquid but in this series of changes we do not pass through two-phase region. If process is carried out at the critical temperature, substance always remains in one phase.
Thus there is continuity between the gaseous and liquid state. The term fluid is used for either a liquid or a gas to recognise this continuity. Thus a liquid can be viewed as a very dense gas. Liquid and gas can be distinguished only when the fluid is below its critical temperature and its pressure and volume lie under the dome, since in that situation liquid and gas are in equilibrium and a surface separating the two phases is visible. In the absence of this surface there is no fundamental way of distinguishing between two states. At critical temperature, liquid passes into gaseous state imperceptibly and continuously; the surface separating two phases disappears . A gas below the critical temperature can be liquified by applying pressure, and is called vapour of the substance. Carbon dioxide gas below its critical temperature is called carbon dioxide vapour.
● He plotted isotherms of carbon dioxide at various temperatures (Fig.).
● Later on it was found that real gases behave in the same manner as carbon dioxide.
● Andrews noticed that at high temperatures isotherms look like that of an ideal gas and the gas cannot be liquified even at very high pressure.
● As the temperature is lowered, shape of the curve changes and data shows considerable deviation from ideal behaviour.
● At `30.98 °C` carbon dioxide remains gas up to `73` atmospheric pressure. (Point `E` in Fig.). At `73` atmospheric pressure, liquid carbon dioxide appears for the first time. The temperature `30.98 °C` is called `text(critical temperature)` (`T_C`) of carbon dioxide. This is the highest temperature at which liquid carbon dioxide is observed. Above this temperature it is gas. Volume of one mole of the gas at critical temperature is called `text(critical volume)` (`V_C`) and pressure at this temperature is called `text(critical pressure)` (`p_C`). The critical temperature, pressure and volume are called critical constants.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Critical temperature of a gas may be defined as that temperature above which it cannot be liquified howsoever high pressure may be applied on the gas.
● Further increase in pressure simply compresses the liquid carbon dioxide and the curve represents the compressibility of the liquid. The steep line represents the isotherm of liquid. Even a slight compression results in steep rise in pressure indicating very low compressibility of the liquid.
● Below `30.98 °C`, the behaviour of the gas on compression is quite different.
● At `21.5 °C`, carbon dioxide remains as a gas only up to point `B`. At point `B`, liquid of a particular volume appears.
● Further compression does not change the pressure. Liquid and gaseous carbon dioxide coexist and further application of pressure results in the condensation of more gas until the point `C` is reached.
● At point `C`, all the gas has been condensed and further application of pressure merely compresses the liquid as shown by steep line.
● A slight compression from volume `V_2` to `V_3` results in steep rise in pressure from `p_2` to `p_3` (Fig. 5.11).
● Below `30.98 °C` (critical temperature) each curve shows the similar trend. Only length of the horizontal line increases at lower temperatures.
● At critical point horizontal portion of the isotherm merges into one point.
● Thus we see that a point like `A` in the Fig. represents gaseous state. A point like `D` represents liquid state and a point under the dome shaped area represents existence of liquid and gaseous carbon dioxide in equilibrium.
● All the gases upon compression at constant temperature (isothermal compression) show the same behaviour as shown by carbon dioxide.
● Also above discussion shows that gases should be cooled below their critical temperature for liquification.
● Critical temperature of a gas is highest temperature at which liquifaction of the gas first occurs.
● Liquifaction of so called permanent gases (i.e., gases which show continuous positive deviation in Z value) requires cooling as well as considerable compression. Compression brings the molecules in close vicinity and cooling slows down the movement of molecules therefore, intermolecular interactions may hold the closely and slowly moving molecules together and the gas liquifies.
● It is possible to change a gas into liquid or a liquid into gas by a process in which always a single phase is present.
● For example in Fig we can move from point `A` to `F` vertically by increasing the temperature, then we can reach the point `G` by compressing the gas at the constant temperature along this isotherm (isotherm at `31.1°C`). The pressure will increase. Now we can move vertically down towards `D` by lowering the temperature. As soon as we cross the point `H` on the critical isotherm we get liquid. We end up with liquid but in this series of changes we do not pass through two-phase region. If process is carried out at the critical temperature, substance always remains in one phase.
Thus there is continuity between the gaseous and liquid state. The term fluid is used for either a liquid or a gas to recognise this continuity. Thus a liquid can be viewed as a very dense gas. Liquid and gas can be distinguished only when the fluid is below its critical temperature and its pressure and volume lie under the dome, since in that situation liquid and gas are in equilibrium and a surface separating the two phases is visible. In the absence of this surface there is no fundamental way of distinguishing between two states. At critical temperature, liquid passes into gaseous state imperceptibly and continuously; the surface separating two phases disappears . A gas below the critical temperature can be liquified by applying pressure, and is called vapour of the substance. Carbon dioxide gas below its critical temperature is called carbon dioxide vapour.

